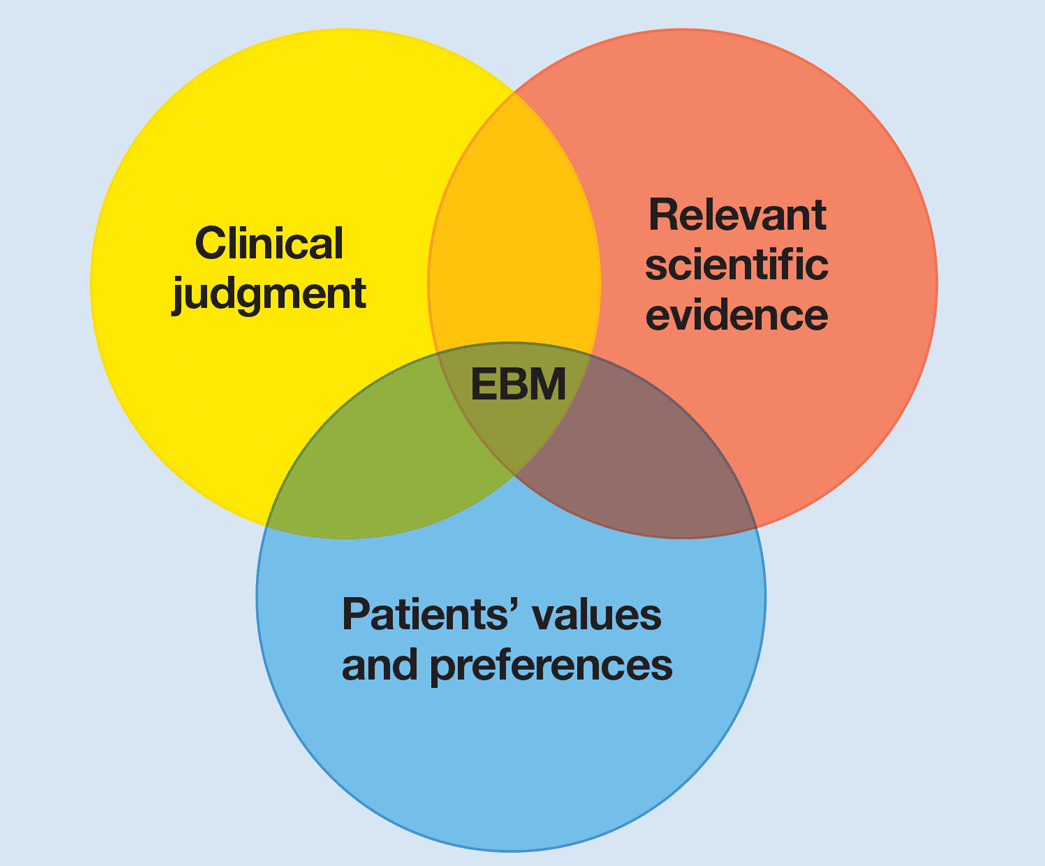
A number of old drugs approved for other diseases are being “repurposed” and tested for their safety and efficacy in COVID-19, in systematic clinical trials. They have emergency approval for use in COVID-19, but most are not yet proven for this use. Some drugs have received approval without adequate testing. Other drugs are being used without getting approval for use in COVID-19. All these drugs are being prescribed widely by doctors, outside of clinical trials.
In this episode, Sandhya Srinivasan, consulting editor for the Indian Journal of Medical Ethics spoke with me. Posted on Suno India.
COVID-19: The need for evidence-based medicine1 min read
Reading Time: < 1 minute