India has the world’s second-largest COVID-19 outbreak. India desperately needs effective treatments. But the way the country’s drug regulator is handling potential therapies concerns many of us. The Drugs Controller General of India (DCGI) has approved several repurposed drugs for ‘restricted emergency use’ for treating the disease. On what basis were these drugs approved? Was …
Covid
Hydroxychloroquine and Covid
My colleagues and I wrote our concern in Lancet Infect Dis about the inappropriate and irrational use of Hydroxychloroquine for preventing and treating Covid in India. We wrote this when the pandemic had just started. The Indian Council of Medical Research, under the Ministry of Health and Family Welfare, has recommended chemoprophylaxis with hydroxychloroquine for …
Covid and Community Transmission
I see three challenges ahead vis-à-vis COVID-19 in India’s rural areas. First, many rural healthcare workers are exhausted and burned out. Second, lifestyle diseases like diabetes, high blood-pressure and heart problems have become more common in rural India in the last decade or so. Third, officials and healthcare workers have to contend with the spectre …
COVID-19 Treatment: Choosing the Right Medicines
The COVID-19 disease is still rapidly evolving and maybe, we were far more cautious earlier than we are today. As we look for treatment, cures and the coronavirus vaccine do we now understand drugs used to treat the novel coronavirus? As patients, do we know enough about these medicines? Are we choosing the right questions …
Covid: Evidence, Ethics and Economics
This afternoon I spoke on several issues that influence our thought processes when we see patients with Covid19- in the community, in the hospital OPD, wards or ICUs. How should we design our therapy? Should we allow ourselves to prescribe untested and unproven therapies because the atmosphere is filled with fear, desperation and panic? What …
Clinical Trials in Covid: Ethics and Practice
Fear. Panic. Desperation. Came Covid and most doctors began to prescribe anti-Covid drugs based not on scientific research, but based on anecdotes, media stories, newspapers, TV channels and promotion of drugs by the drug industry. The virus pushed the Evidence-based medicine to the back seat. Physicians were either reluctant to— or didn’t know how to— …
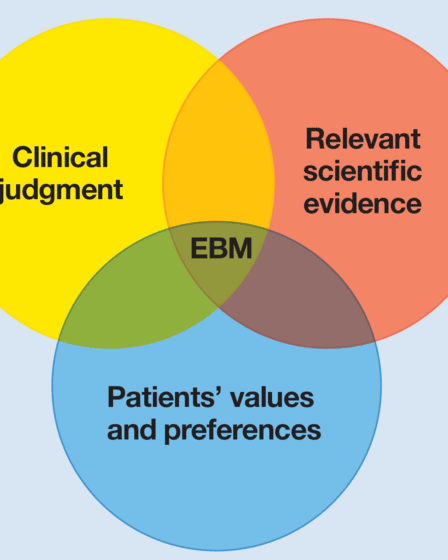
COVID-19: The need for evidence-based medicine
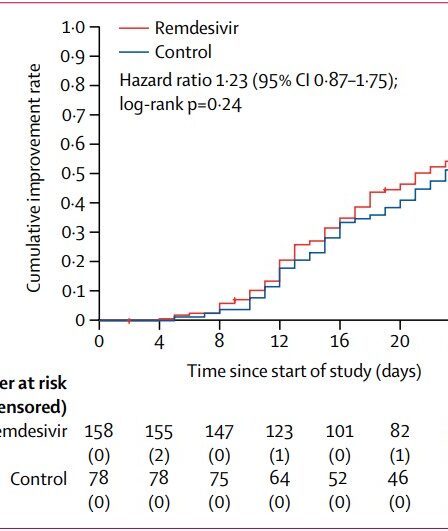
A number of old drugs approved for other diseases are being “repurposed” and tested for their safety and efficacy in COVID-19, in systematic clinical trials. They have emergency approval for use in COVID-19, but most are not yet proven for this use. Some drugs have received approval without adequate testing. Other drugs are being used …
Covid : Defining the Research Priorities?
Here is a YouTube link to the PowerPoint presentation.
Covid: Clinical Care and Research
Here is a link to the PowerPoint presentation on YouTube :
Covid: Practical Hospital Level Care and Interventions
On 13 July 2020, I spoke on how should we organise hospital level care and use evidence based interventions to help our Covid patients. Dr Cliff Lane and Gagandeep Kang also spoke in this session. Here is a YouTube link to my PowerPoint presentation.