Fear. Panic. Desperation. Came Covid and most doctors began to prescribe anti-Covid drugs based not on scientific research, but based on anecdotes, media stories, newspapers, TV channels and promotion of drugs by the drug industry. The virus pushed the Evidence-based medicine to the back seat. Physicians were either reluctant to— or didn’t know how to— …
EBM
COVID-19: The need for evidence-based medicine
A number of old drugs approved for other diseases are being “repurposed” and tested for their safety and efficacy in COVID-19, in systematic clinical trials. They have emergency approval for use in COVID-19, but most are not yet proven for this use. Some drugs have received approval without adequate testing. Other drugs are being used …
Covid: Clinical Care and Research
Here is a link to the PowerPoint presentation on YouTube :
Covid: Practical Hospital Level Care and Interventions
On 13 July 2020, I spoke on how should we organise hospital level care and use evidence based interventions to help our Covid patients. Dr Cliff Lane and Gagandeep Kang also spoke in this session. Here is a YouTube link to my PowerPoint presentation.
Covid and Ayurveda
I criticised Patanjali Ayurveda’s claimed cure for COVID-19 for making unsubstantiated claims of efficacy. The drugs it is offering are untested and unproven. They haven’t proven their mettle in a well-conducted adequately sized randomized controlled trial. The proponents of Ayurveda, however, ask – can Ayurveda, or alternative medicine in general, be evaluated in the same …
Ethical Challenges of Research in a Pandemic
The pressure for an effective treatment or vaccine for COVID-19 is high. There are more than 2,000 studies on COVID-19 across the world; many of these are trials on humans to develop vaccines, and to test the efficacy of drugs for this new disease. The urgency of this pandemic presents new, acute ethical challenges in …
Covid 19: We need well-designed clinical trials
On June 23, Patanjali Ayurved claimed that its preparations, ‘Coronil’ would cure COVID-19 in just a week. Scientists, researchers, physicians and media registered their strong protest and expressed robust disbelief for the outrageous and misleading ads. The company claimed that their medicine was tested in a randomised controlled trial (RCT) among COVID-19 positive patients and …
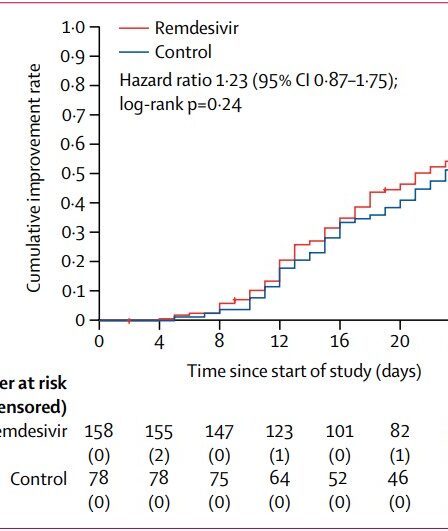
The many questions about Favipiravir
The Drugs Controller General of India (DCGI) has approved Glenmark Pharmaceuticals, an Indian pharmaceutical company, to sell generic versions of Favipiravir for the treatment of COVID-19. This drug, originally invented by a Japanese company, was meant to treat influenza. After the outbreak of COVID-19, doctors in China and Russia started using it to treat COVID-19 …
Clinical Research during Covid Era
On 18 June 2020, Dr. C. S. Pramesh, the Director of the Tata Memorial Hospital texted me a message and then called me asking if I would be willing to do a talk on Covid and research during Pandemic. A day after. Although I had little time to prepare, I didn’t blink an eyelid to …
Hydroxychloroquine and COVID-19: Can we go back to science?
Hydroxychloroquine (HCQ) has been the biggest buzzword across the world lately. Until December 2019, this was a meek drug, used mainly by rheumatologists, internists, and dermatologists for inflammatory diseases. Its cousin Chloroquine has been the most popular drug for malaria. We have known and used these drugs for decades. The antiviral properties of these drugs …