Fear. Panic. Desperation. Came Covid and most doctors began to prescribe anti-Covid drugs based not on scientific research, but based on anecdotes, media stories, newspapers, TV channels and promotion of drugs by the drug industry.
The virus pushed the Evidence-based medicine to the back seat. Physicians were either reluctant to— or didn’t know how to— use the best evidence currently available on safety and efficacy in making decisions on treatment choices for their patients.
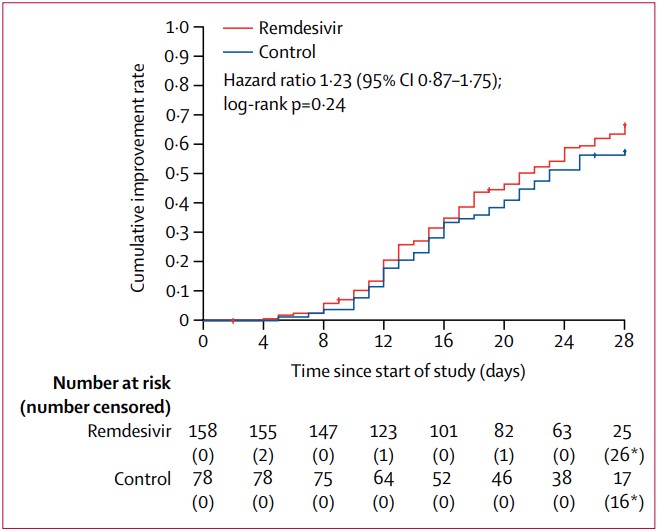
During the early part of the pandemic, some of the early trials were rushed, leading to studies that were badly designed, poorly conducted or had too few patients. As a result, initial evidence of the efficacy of some Covid-19 treatments— Hydroxychloroqune and Remdesivir, as an example— could not be replicated. These drugs were already in widespread use by then, and physicians were reluctant to change to proven efficacious alternatives.
“An ongoing pandemic justifies leeway in generation and interpretation of evidence in the interest of public health. However, all scientific reasoning cannot be abandoned citing desperate times.” We wrote in Lancet Infectious Disease. “If this is not done,” we had warned, “If this is not done, the risk–benefit assessment would be skewed, adverse events accepted as collateral damage, and a drug accepted provisionally in a time of crisis could become commonplace as standard of care for a long time to come.”
Dr. Premanath Kotur and Dr. Mahalakshmi from the VN and Aarupadai Veedu Medical College & Hospital (AVMC) Puducherry hosted a series of online seminars during this period. They asked me if I could do a 45-minute-long seminar on Clinical Trials in Covid: Ethics and Practice. I agreed.