Molnupiravir. Another drug that has created a lot of hype or hope among public. But does it really deliver what it promises?
Blatant lies. Unsubstantiated tall claims. Not a word on potential risks. #Molnupiravir, says the flier, also reduces post-Covid anxiety, depression and fatigue. #Omicron is growing, and so are the product claims made by #drugcompanies— taller every day. pic.twitter.com/rIn0Sr9jhU
— SP Kalantri (@spkalantri) January 8, 2022
If 40 Covid + at risk adults swallow 40 pills of #Molnupiravir over 120 hours, 1 of them-who, nobody can predict- might be spared of hospital admission.
— SP Kalantri (@spkalantri) January 16, 2022
Benefit of an antiviral explained in plain English.
Five new therapies have caught the fancy of physicians, worldwide, for the treatment of mild-moderately ill, at-risk outpatients with Covid19.
— SP Kalantri (@spkalantri) January 2, 2022
Which drug to use? I have summarised the efficacy of each drug— #Paxlovid, #Sotrovimab, #Remdesivir, #Molnupiravir and #Fluvoxamine. pic.twitter.com/MLUROBfOKw
#Molnupiravir has created an exciting vibe
— SP Kalantri (@spkalantri) January 7, 2022
And has caught the fancy of my tribe
I shouldn’t be too hasty
Think I must of patients’ safety
And resist the temptation to prescribe
Merck’s #Molnupiravir should remind everyone of Merck’s #Vioxx—then a popular painkiller. Before it was withdrawn in 2004, it might have killed 56000 & left 140000 with heart disease.
— SP Kalantri (@spkalantri) January 4, 2022
It takes time for the drug to show its true colours. And the Pharma downplays the dark story.
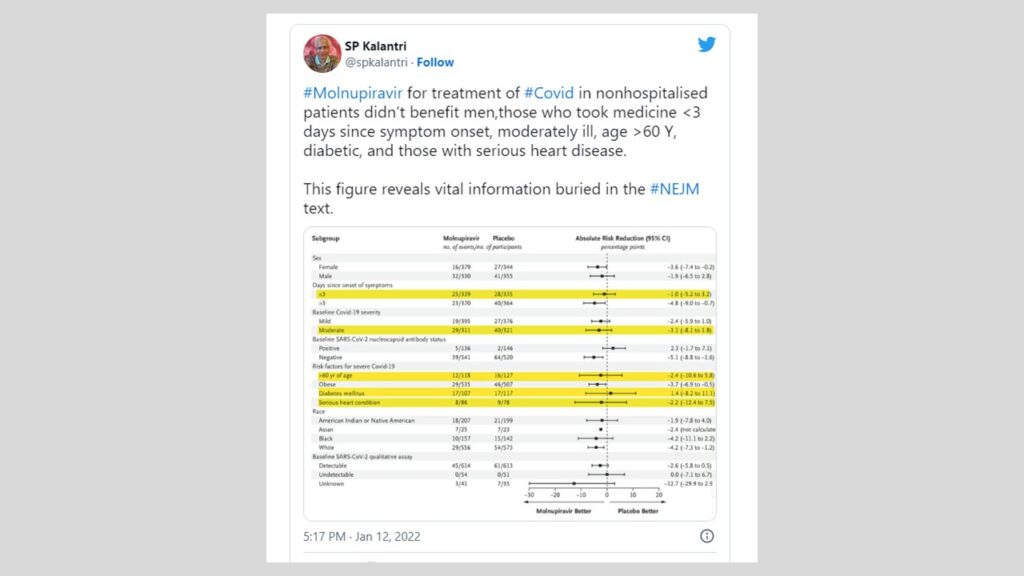
#Molnupiravir for treatment of #Covid in nonhospitalised patients didn’t benefit men,those who took medicine <3 days since symptom onset, moderately ill, age >60 Y, diabetic, and those with serious heart disease.
— SP Kalantri (@spkalantri) January 12, 2022
This figure reveals vital information buried in the #NEJM text. pic.twitter.com/Z95kAWkk7b
#Favipiravir in 2020. #Molnupiravir in 2022.
— SP Kalantri (@spkalantri) January 4, 2022
The more things change, the more they stay the same.
▶️Patient:
— SP Kalantri (@spkalantri) January 4, 2022
I am 65. I have high BP and diabetes. I tested positive for Covid two days back. Will #Molnupiravir benefit me?
▶️Honest answer:
If we treat 3⃣3⃣patients like you, we might save 1⃣ patient from getting admitted to the hospital. Who of the 33—we don’t know.
NICE removed #budesonide from recommended #COVID19 treatments because it failed to reduce hospital admissions or deaths—odds ratio 0·75; 95% CI, 0·55-1·03.
— SP Kalantri (@spkalantri) January 11, 2022
Did #Molnupiravir perform better? Its numbers are almost identical—hazard ratio, 0.69; 95% CI, 0.48 to 1.01.
Time to go?
When I explained to a hypothetical 40- Y- old patient the benefits and risks of #Molnupiravir medicine for reducing the risk of disease pogression in mild #COVID19, the person brooded over the options, and said,
— SP Kalantri (@spkalantri) January 7, 2022
"और कोई दुसरा माल़ दिखाइये।"
[1] Sotrovimab [2] Bamlanivimab + Etesevimab [3] Casirivimab + Imdevimab and [4] Molnupiravir.
— SP Kalantri (@spkalantri) December 25, 2021
Novel therapies to treat #COVID19 .
I wonder if I got their spellings right—I can’t even pronounce them correctly!
1/5
— SP Kalantri (@spkalantri) July 10, 2021
Hetero press release on Molnupiravir says the drug makes the patients with mild Covid tend to feel better when they swallow the pill.
The 3-page press release devotes 2 pages to the contract, company and the drug chemistry. The trial and study results get half-page each.
2/5
— SP Kalantri (@spkalantri) July 10, 2021
The press release does not say a word on trial design, participant eligibility criteria, settings and locations, sample size, randomisation (sequence generation, allocation concealment, who enrolled and who assigned participants to treatments) or blinding.
3/5
— SP Kalantri (@spkalantri) July 10, 2021
Since no patient in the study—either group—died, was put on a mechanical ventilator, went to the ICU or had prolonged high flow oxygen therapy, the study captured soft end points. Outcomes that may not matter to patients.
4/5
— SP Kalantri (@spkalantri) July 10, 2021
Virus clearance at day 5 is a surrogate end point. Who cares? The crucial question is did the drug stop the disease progression- ICU admission, ventilators or death?
And a difference that is statistically significant might not be clinically meaningful.
5/5
— SP Kalantri (@spkalantri) July 10, 2021
The study says that it used WHO clinical progression scale. The original scale was a 10-point ordinal scale. RCTs have also used 7 and 8 point scales.
The press release gives no details, nor the break-up of patients on each point of the scale. pic.twitter.com/yf130RzZLn
6/n
— SP Kalantri (@spkalantri) July 10, 2021
Why hospitalsiation shouldn't be an outcome measure? Several factors account for it. The fear of the disease makes mildly sick patients seek admision; families push them in the hospital and private hospitals keep their private room doors ajar to the paying patients.
Before physicians prescribe #Molnupiravir to at-risk people, they must ensure that the woman is not pregnant and uses contraception during and 4 days after completion of the therapy.
— SP Kalantri (@spkalantri) January 4, 2022
We don't know if the drug could harm the fetus.
How do we ensure its safety in rural India?
Many drug trials on #Covid19—e.g. #Molnupiravir—were conducted during a period when hospitalisation rate was 21%.
— SP Kalantri (@spkalantri) January 23, 2022
The current rate is 3%.
When the baseline risk of hospitalisation has fallen so much, how do we apply the results of those studies to the presently ill patients?
#Molnupiravir has an edge over monoclonal antibodies in the treatment of Covid19 patients.
— SP Kalantri (@spkalantri) December 24, 2021
Unlike the antibodies, its action is independent of mutations in the spike protein.
Hopefully it should work against the newer variants.
The 3 most common SARS-CoV-2 variants in the #Molnupiravir trial were delta [58%], mu [20%], and gamma [11%]. #Omicron variant was yet to be discovered then.
— SP Kalantri (@spkalantri) January 12, 2022
What makes us use Monupiravir for Omicron, against whom it has never been tested?
Status: Vaccinated. Diabetic. Omicron infected. Height 1.65 meter; weight 60 kg (BMI 22).
— SP Kalantri (@spkalantri) January 16, 2022
Q: Will #Molnupiravir work in such a patient?
A: The @NEJM study didn't enrol vaccinated + those with omicron. 74% study patients were obese—BMI of 30. The drug did not work in diabetic.
Now it is #Merck vs. #Pfizer.
— SP Kalantri (@spkalantri) November 5, 2021
Close on the heels of Merck’s Molnupiravir that promises to reduce the risk of Covid hospitalization or deaths by 50%, Pfizer says that its Paxlovid is a cut above—it reduces the risk by 89%.
Let us see how these drugs play out in the real world.
Of note, #Molnupiravir was found to be no better than placebo in several subgroups—patients who had had #COVID19 in the past, those with low baseline viral load, and those with diabetes.
— SP Kalantri (@spkalantri) December 24, 2021
Also its eficacy among vaccinated persons is unknown.
Important limitations.
In the 5 recent trials designed to test the efficacy of #COVID19 drugs— #Paxlovid, #Sotrovimab, #remdesivir, #Molnupiravir and #Fluvoxamine— for mild to moderately ill, at-risk Covid patients, of the 4849 patients, 34 died(in both arms of the study).
— SP Kalantri (@spkalantri) January 7, 2022
7 deaths per 1000 patients. pic.twitter.com/C6cJF87CNe
The baseline risk of #Omicron-induced #COVID19 needing hospitalisation, oxygen support, mechanical ventilation and ICU care is very low.
— SP Kalantri (@spkalantri) January 14, 2022
Given this, why should we deploy drugs to reduce this drug further—drugs with meagre benefits and substantial potential risk?#Molnupiravir
We must ask why #Molnupiravir hands a raw deal to children. And to couple trying to conceive & to women who are already pregnant.
— SP Kalantri (@spkalantri) January 5, 2022
Why #Paxlovid is not kind to those on heart medicines.
And why #Omicron defies some monoclonal antibodies.
There is more to drugs than meets the eye
Surprises never cease!
— SP Kalantri (@spkalantri) January 19, 2022
The revised #COVID19 protocol of the state recommends #Favipiravir for mild cases, #Molnupiravir (“If available”) for mildly ill with no co-morbidity, a battery of blood tests for moderately ill, and CT chest on day five.
The #Molnupiravir for Covid19 trial [MOVe-OUT] enrolled 332 patients with low viral loads.
— SP Kalantri (@spkalantri) January 12, 2022
10 of 160 such patients put on the drug required hospitalisation compared to 9 of 162 on usual therapy.
Where is the evidence that it works in patients with low vial loads?
This is the efficacy of #Molnupiravir against Covid related hospitalisation.
— SP Kalantri (@spkalantri) January 13, 2022
Hazard ratio, 0.69; 95% CI, 0.48 to 1.01
And this is the effectivity of #BoosterDose Covid vaccine.
Hazard ratio, 0.11; 95% CI, 0.06 to 0.21
The numbers speak for themselves.
@paimadhu
▶️Benefit
— SP Kalantri (@spkalantri) January 4, 2022
If 100 at-risk persons test Covid+,🔟will need hospital admission.
If 100 such persons swallow 40 pills of #Molnupiravir for 5 days within 3 days of disease onset, 7⃣will be admitted to the hospital.
▶️Risk:
We are not sure if the drug is safe—long term.
Small benefits; large potential risk. How many patients shall be willing to take the drug after they are fully informed about the effectivity and (lack of) safety data? #Molnupiravir https://t.co/kZTx85keFv
— SP Kalantri (@spkalantri) January 6, 2022
Days are not far when we shall see #Paxlovid /Ritonavir+ #Molnupiravir+ Monoclonal Antibodies+ #Remdesivir combo used to treat Covid+ high risk individuals.
— SP Kalantri (@spkalantri) January 1, 2022
Using four drugs to treat a mildly ill Covid+ patient—this would not only be irrational but also a dreadful idea.
The #Molnupiravir for Covid19 trial [MOVe-OUT] reported in #NEJM enrolled 47 Asians.
— SP Kalantri (@spkalantri) January 12, 2022
Seven of 25 patients put on the drug went to the hospital compared to six of 22 on usual therapy.
Where is the evidence that it works in Indians?
#Molnupiravir, the oral drug that has recently jumped on Covid bandwagon has only been tested—and found effective—in vaccine naïve Covid19 infected individuals.
— SP Kalantri (@spkalantri) December 24, 2021
Will it benefit as well those who acquire Covid19 after vaccination? We don’t know.
#Favipiravir, Itolizumab, 2DG and #Molnupiravir. The fourth time that the ICMR task force has failed to see eye-to-eye with CDSCO. And decided to exclude Molnupiravir in the Covid-19 management guidelines.@CDSCO_INDIA_INF proposes, @ICMRDELHI disposes!
— SP Kalantri (@spkalantri) January 10, 2022
Because the effect size is small—and imprecise—it’s fair to say that most people who swallow #Molnupiravir tablets do not obtain purported benefit from the drug—staying at home and avoiding hospitalisation.
— SP Kalantri (@spkalantri) January 10, 2022
(hazard ratio, 0.69; 95% CI, 0.48 to 1.01) #NEJM 16 Dec 2021 study pic.twitter.com/BIeVuzwJrA
Several patient characteristics in #Molnupiravir RCT merit attention.
— SP Kalantri (@spkalantri) January 11, 2022
The enrolled patients were unvaccinated, did not suffer past Covid infection and were not infected with the #Omicron variant.
A sixth of study patients had #diabetes. The drug didn't work in this group. 1/2
Also it did not work in those with low baseline viral loads.
— SP Kalantri (@spkalantri) January 11, 2022
Patients who were likely to get hospitalized within 2 days of enrollment were excluded.
How do we apply these results to #Covid patients in India in the present wave? 2/2
Is a polypill—fixed-drug combination in one pill—for the outpatients with early COVID-19 in the offing?
— SP Kalantri (@spkalantri) January 2, 2022
Might drug companies take a cue from cardiology and start marketing a polypill (#fluvoxamine+#molnupiravir+ #Paxlovid?
Because that is where the money—and irrationality—is.
When the baseline risk is low; the benefits of therapy, small; and the potential risk, large; shouldn’t physicians choose a drug only if the odds of benefit outweigh the odds of harm? #Molnupiravir in #COVID19
— SP Kalantri (@spkalantri) January 10, 2022
If we put 100 at-risk outpatients with #COVID19 on #Molnupiravir, the drug might reduce the risk of hospitalisation by 31%—our best estimate.
— SP Kalantri (@spkalantri) January 10, 2022
The risk reduction cuts both ways—it could reduce the risk by 52%. It may also up the risk by 1%.
The problem with the @NEJM #Molnupiravir trial in #Covid19 is that the effect size is small at 3% absolute risk reduction (we need to treat 33 patients to prevent one hospitalisation) and the results are fragile—with 95% CI crossing 1. (hazard ratio, 0.69; 95% CI, 0.48 to 1.01)
— SP Kalantri (@spkalantri) January 10, 2022
Might #Molnupiravir study results not apply to a third of Indians who are fully vaccinated?
— SP Kalantri (@spkalantri) January 16, 2022
And those who have had Covid in the past?
And those currently fighting the #Omicron variant?
Pfizer's #Paxlovid vs. Merck and Ridgeback's #Molnupiravir.
— SP Kalantri (@spkalantri) January 2, 2022
Will it, won't it… the match is getting interesting!
The #Molnupiravir for Covid19 trial [MOVe-OUT] enrolled 164 patients with serious heart disease. 8 of 86 patients put on drug went to the hospital compared to 9 of 78 on usual therapy.
— SP Kalantri (@spkalantri) January 12, 2022
Where is the evidence that it works in heart disease—a risk factor for progression of disease?
Fully agree. This gudileine collects and critically appraisesthe evidence and help the doctos apply it at the patient's bedside.
— SP Kalantri (@spkalantri) January 5, 2022
Am looking forward to updates on recent anti-Covid theapies for at-risk outpatients— Paxlovid, Molnupiravir, Remdesivir, Fluvoxamine and Sotromivab.
1. The 22 Dec @NEJM study paints #Remdesivir with rich and bright colours—making one believe that the drug works wonderfully well in infected outpatients at high risk for severe Covid19.
— SP Kalantri (@spkalantri) December 23, 2021
2. Let me summarise the important limitations of the trial.
— SP Kalantri (@spkalantri) December 23, 2021
First, the inclusion criteria. Because only 2.5% of the study patients were Asians, we cannot generalize these data to the Asians. Of note, Remdesivir did not work in the Hispanic population in this study.
3. Participants were excluded if they were receiving or were expected to receive supplemental oxygen at the time of screening.
— SP Kalantri (@spkalantri) December 23, 2021
Patients were also ineligible if they had received a SARS-CoV-2 vaccine. This criterion alone would exclude a large number of patients.
4. The last patient was enrolled on April 8, 2021—before the #Delta variant of SARS-CoV-2 emerged as the dominant circulating strain. Can we apply the study results to the delta or Omicron variants?
— SP Kalantri (@spkalantri) December 23, 2021
5. 1 of 20 patients who received Remdesivir was spared hospitalization. Looks impressive but note that no patient died in either group by day 28, an important outcome measure. The 2 lines in the Kaplan–Meier graph do diverge, but we must read between the lines.
— SP Kalantri (@spkalantri) December 23, 2021
6. The most common adverse events in both groups were nausea, headache, and cough. These are classical Covid symptoms—what made the researchers label them as the adverse events?
— SP Kalantri (@spkalantri) December 23, 2021
7. Despite its perceived efficacy, remdesivir failed to reduce the upper airway viral load. This contrasts with monoclonal antibodies #molnupiravir, both of which reduce viral burden rapidly.
— SP Kalantri (@spkalantri) December 23, 2021
8. And there is a practical challenge. Only a sixth of study patients received infusion at home. How do we ensure home-based access of Remdesivir to an infected person in resource limited setting?
— SP Kalantri (@spkalantri) December 23, 2021
9. Researchers have faced huge problems in injecting single-dose monoclonal AB—how can we identify, infuse the drug and monitor outpatients for a 3-day outpatient intravenous remdesivir therapy?
— SP Kalantri (@spkalantri) December 23, 2021
10. The trial had to be stopped half way through because there were ethical concerns regarding assigning patients to when the vaccination rates rose and monoclonal antibodies became available. These issues are much more relevant today than in early 2021.
— SP Kalantri (@spkalantri) December 23, 2021
11. Authors plead that outpatient remdesivir could serve vaccine-naïve persons. In resource limited settings, can those who are unable to access vaccine access remdesivir? Bread vs Cake?
— SP Kalantri (@spkalantri) December 23, 2021
12.The authors selectively hype ACTT-1 and SIMPLE trials and downplay Wuhan and the WHO Solidarity trial.
— SP Kalantri (@spkalantri) December 23, 2021
So, a pharma sponsored trial. Trying to thrust Remdesevir back into the limelight. Much ado about nothing? END.
This is a sentence from the NEJM 16 dec 2021 original clinical trial:"The rate of hospitalization or death through day 29 was approximately 31% lower with molnupiravir than with placebo (hazard ratio, 0.69; 95% CI, 0.48 to 1.01)"
— SP Kalantri (@spkalantri) January 15, 2022
Why did the 95% CIs cross 1? What does it mean?
The #Molnupiravir for Covid19 trial [MOVe-OUT] enrolled 224 patients with diabetes. 17 of 107 patients put on drug went to the hospital compared to 17 of 117 on usual therapy.
— SP Kalantri (@spkalantri) January 12, 2022
Where is the evidence that it works in adults with diabetes—a risk factor for progression of disease?